Prescription Policy Reform for Pakistan
Abstract
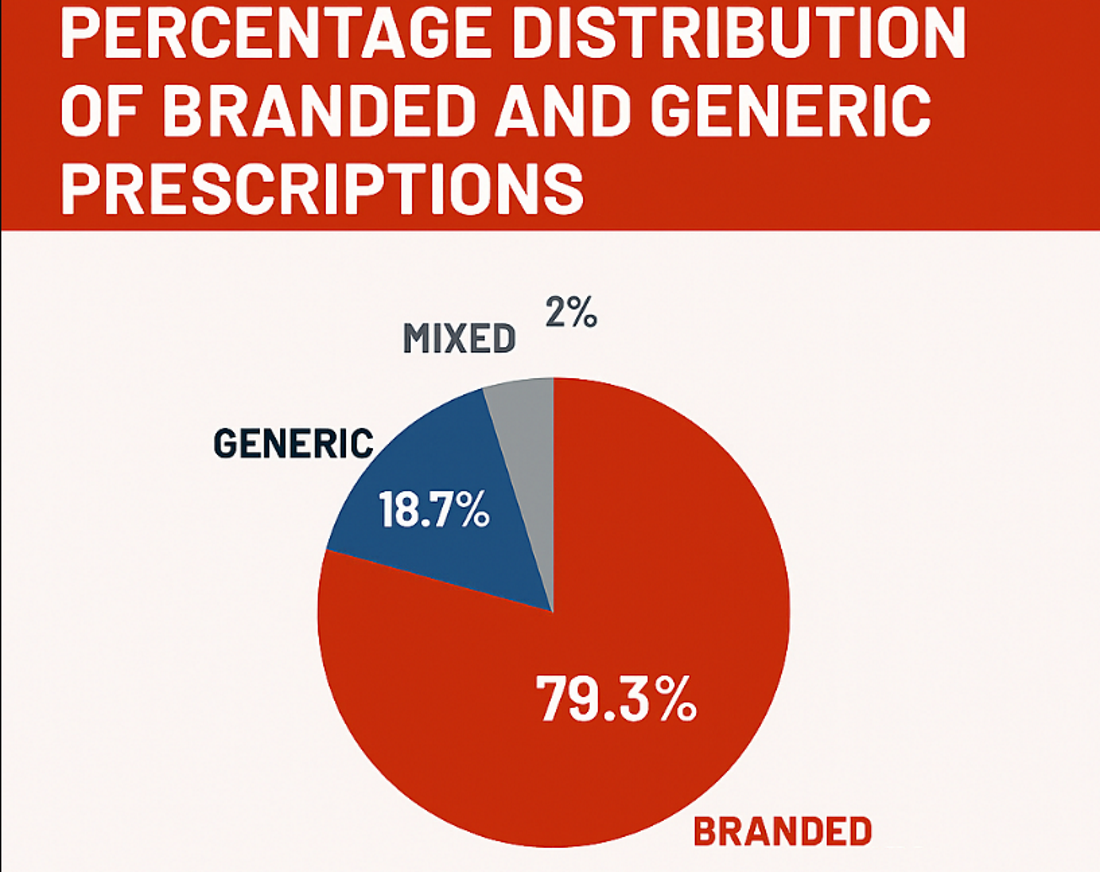
Background: Pakistan’s healthcare system faces rising costs and inequitable access to medicines, with branded pharmaceuticals dominating prescriptions despite the availability of less expensive generics. Out-of-pocket medicine expenditure exceeds 60% of total health spending, placing an unsustainable burden on patients.
Objective: To evaluate the prescription dynamics between branded and generic medicines in Pakistan, identify policy and regulatory gaps, and propose a strategic framework to promote quality-assured generic prescribing as a national cost-containment measure.
Methods: A mixed-methods study design is proposed, combining quantitative analysis of prescription data from tertiary hospitals with qualitative interviews of prescribers, pharmacists, patients, and policymakers. Regulatory documents from the Drug Regulatory Authority of Pakistan (DRAP) and comparative regional policies will be analyzed to inform recommendations.
Results: Preliminary review indicates limited generic uptake due to inadequate bioequivalence enforcement, physician skepticism, and aggressive pharmaceutical marketing. Evidence from comparable low- and middle-income countries (LMICs) shows that mandatory generic prescribing, coupled with strong quality assurance, can lower national drug costs by 25–40%.
Conclusions: Generic promotion through policy reform, improved regulatory oversight, and physician education can significantly reduce healthcare costs and improve medicine accessibility. A phased roadmap integrating DRAP-led certification, prescriber incentives, and public awareness initiatives is essential for sustainable reform.
Citation: (formatted-apa)
License
Copyright (c) 2025 Dr. Hassan Khan (Author)
This work is licensed under a Creative Commons Attribution 4.0 International License.